Ethics, Compliance and Audit Services
Foreign Influence
NEW 08.18.2021 Global Engagement One Page Information Sheet
Faculty One Page Information on Global Engagement (pdf)

- Introduction
- Undue Foreign Influences on Research Integrity
- Recently Issued Communications, Guidance, Regulations, and Policies
- National Institutes of Health [NIH]
- National Science Foundation [NSF]
- Department of Energy [DOE]
- Department of Defense [DOD]
- Department of State [DOS]
- National Aeronautics and Space Administration [NASA]
- Senate and NIH Correspondence
- Senate Committee on Finance
- Congress
- Department of Education
- Department of Commerce
- White House Executive Orders
- Enforcement Activities
- Office of Science Technology Policy
- DHHS Office of Inspector General
- Higher Education Organizations Science and Security Resources
- Federal Law Enforcement Activity
- Transparency in Disclosures of Foreign Relationships and Activities
- UC and Sponsor Policies
- FAQs
- Links to UC Campus Resources and Websites
- Links to other Institutions of Higher Education Foreign Influence websites/guidance
- Contact Information
Introduction
Since early 2018, we have observed heightened awareness and increased activity related to the issue of foreign influence in academia within the federal government and amongst our peer institutions. Federal funding agencies have issued new requirements and guidance, federal law enforcement agencies have increased prosecutorial activity, and Congress has signed new legislation and sought information on how the academic research community is responding to this evolving issue.
The University of California recognizes that these issues are significant. President Janet Napolitano addressed the essence of these concerns in her letter to the Chancellors and the Lawrence Berkeley National Laboratory Director on February 7, 2019. In her letter, she requested that the Office of Ethics, Compliance, and Audit Services (ECAS) design a compliance plan to address these issues and in support of the University’s core mission and commitment to openness in research and international research collaborations. Collectively, UC performs nearly 1/10th of the nation's academic research and receives over $3 billion annually in federal research awards. International collaborations play a crucial part in much of our research. The University of California's commitment to global engagement is inextricable from our core values.
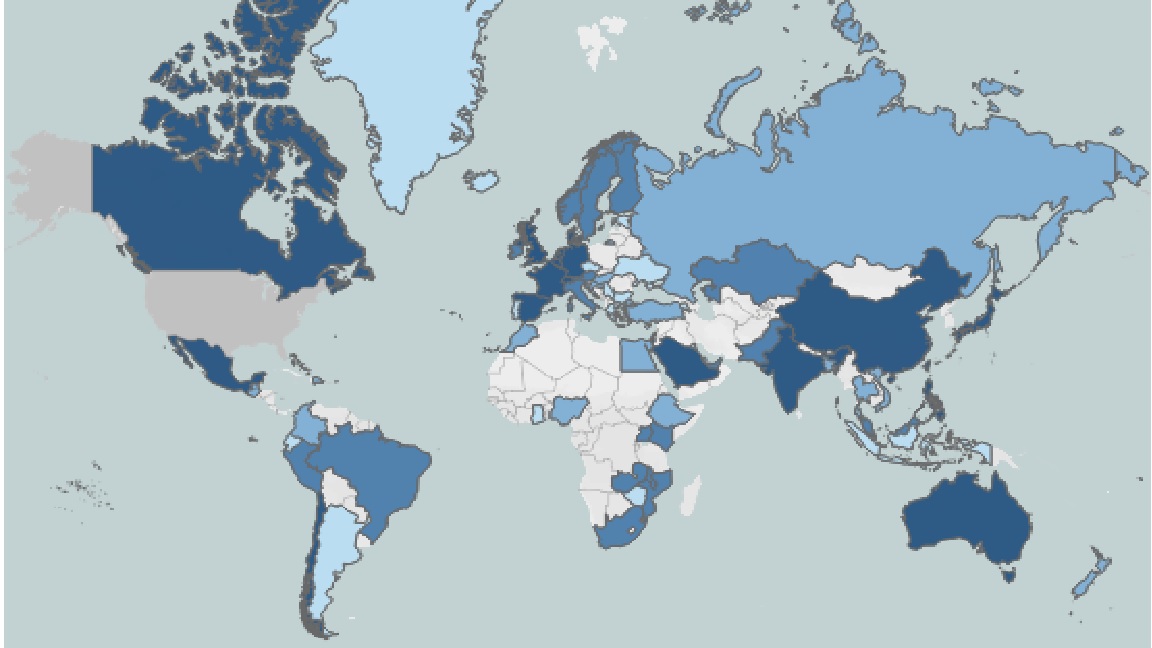
Map of International Sponsors of UC Research
Our compliance plan, detailed more fully below in the FAQs section, is broken into four categories: (i) training and awareness, (ii) compliance assessments, (iii) internal audits, and (iv) investigative protocols. This Foreign Influence resource website is the first in a series of training and awareness tools developed for the UC research community to better understand the evolving compliance landscape.
Undue Foreign Influences on Research Integrity
Federal agencies and policymakers have expressed concern that foreign entities may be using the academic research enterprise in an attempt to compromise the United States’ economic competitiveness and national security. Federal funding agencies have sought to clarify longstanding policies and issued new guidance. Federal law enforcement agencies have increased prosecutorial activity in unison with the federal funding agencies, and Congress has signed new legislation and sought information on how the academic research community is responding to this evolving issue.
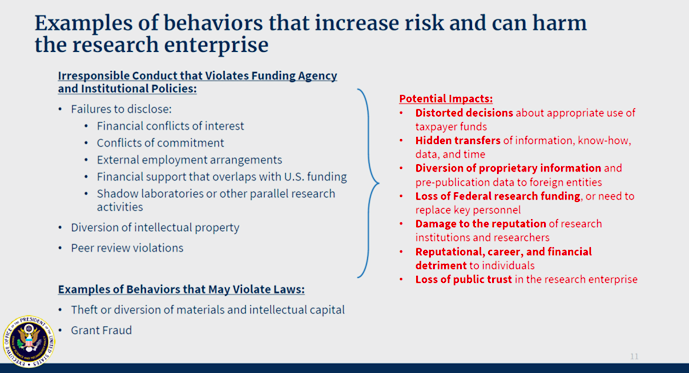
The primary thrust of the USG’s concerns fall into four buckets:
- Peer review violations
- Failure to disclose substantial foreign resources:
- Foreign employment arrangements
- Foreign grant support that creates problems with overlap, or over-commitment
- Non-disclosure of substantial foreign research support
- free labor (visiting scholar/student funded by a foreign source)
- Talents awards
- Foreign grants – Hidden transfers of information, know-how, data, person-time
- Failure to disclose significant foreign financial Conflict of Interest:
- Equity in foreign companies
- Foreign patents that leverage US tax-payer funded work
- Compliance with Regulatory Requirements: U.S. Export Control laws and regulations establish a set of requirements for the transfer of technology and data to foreign countries and/or foreign nationals in the U.S and sanctions from the Office of Foreign Assets Control (OFAC) restrict interactions with individuals or entities on the sanctions list.
The Office of Science and Technology Policy (OSTP) provides some examples of behaviors that can increase risk and harm the research enterprise.
Communications, Guidance, Regulations, and Policies from Federal Agencies and USG
The federal funding agencies, Congress, the federal agencies, and the White House have all issued some form of communication, guidance, new regulations or policies related to dealing with the issue of undue foreign influences on research integrity. Likewise, Higher Education Organizations such as the American Council on Education have issued briefings and position papers on this topic. Below you will find a list of source documents from each agency or organization.
National Institutes of Health [NIH]
In August 2018, Director of the National Institutes of Health (NIH) Francis Collins issued a Foreign Influence Letter to Grantees and testified to the Senate Health, Education, Labor, and Pensions Committee regarding concerns about systematic programs of foreign influence in U.S. research.
NIH Protecting U.S. Biomedical Intellectual Innovation WebPage [NEW 7.16.2020]
NIH and the biomedical research enterprise have a long history of International collaborations with rules of engagement that allow science to advance while also protecting intellectual capital and proprietary information of the participating countries. These rules of engagement also are designed to limit bias in the design, conduct, and reporting of NIH-supported research. This page describes actions that NIH, institutions, and researchers can take to protect U.S. biomedical intellectual innovation. The principles described here align with those announced by the White House's Office and Science and Technology Policy in June 2020.
NIH Director Collins Dear Colleague Foreign Influence Letter
In December 2018 and June 2019, the NIH Advisory Committee to the Director (ACD) released a report entitled ACD Working Group for Foreign Influences on Research Integrity identifying recommendations around communication and awareness; risk mitigation; and monitoring, actions, and consequences.
NIH Advisory Committee to the Director Foreign Influence Report June 2019
NIH Advisory Committee to the Director Foreign Influence Report December 2018
In July, NIH issued NOT-OD-19-114 clarifying and reminding the research community of NIH policies on Other Support and Foreign Components.
Frequently Asked Questions Other Support and Foreign Components Initial Posting: June 19, 2019
National Science Foundation [NSF]
NSF Commissioned JASON Report
NSF commissioned the report to enhance the agency’s understanding of the threats to basic research posed by foreign governments that have taken actions that violate the principles of scientific ethics and research integrity. With the official receipt of the report, NSF will now begin the process of analyzing its findings and recommendations.
NEWS ALERT: US National Science Foundation reveals first details on foreign-influence investigations [NEW July 2020] https://www.nature.com/articles/d41586-020-02051-8
“The US National Science Foundation (NSF) has for the first time released figures on the actions it has taken against researchers found to have violated rules on the disclosure of foreign ties. Since 2018, the agency has reassigned, suspended or terminated grants, forced institutions to return funds or barred researchers from applying for future funding in 16–20 cases in which rules weren’t followed, according to Rebecca Keiser, the agency’s first chief of research security strategy and policy. All of these were cases in which the NSF’s Office of Inspector General, an independent body responsible for oversight of the agency and its grant recipients, had investigated and made recommendations on how to handle sanctions. Separately, the inspector-general referred an undisclosed number of criminal and civil cases involving fraud and nondisclosure to the US Department of Justice. Furthermore, in the past two months, seven universities have contacted the NSF directly with information on faculty members who might have violated rules.”
NSF- Statement of The National Science Board on Security and Science October 23, 2018 NSB-2018-42
Proposal & Award Policies & Procedures Guide January 2020
NSF Dear Colleague Research Protection Letter 7.11.19
The NSF Dear Colleague letter outlined a few steps it is taking to mitigate the risks in concert with other agencies and stakeholders. Highlights from the letter:
- Citizenship Requirements
- To ensure that NSF is applying consistent standards to all staff members, each of whom has access to sensitive merit review and other information, we issued a requirement in April 2018 that rotators working onsite at NSF must be U.S. citizens or have applied for U.S. citizenship.
- Disclosure Requirements
- Since 1978, NSF has required senior project personnel on proposals to disclose all sources of support, both foreign and domestic.
- Proposal and Award Policies and Procedures Guide
- A renewed effort is now underway to ensure that existing requirements to disclose current and pending support information are known, understood, and followed.
- For example, in May, we published in the Federal Register a proposed clarification of our proposal disclosure requirements (open for public comment through July 29). Our draft NSF Proposal and Award Policies and Procedures Guide includes clarifications regarding reporting requirements for both current and pending support and professional appointments.
- To streamline the process for providing these disclosures to NSF, we are proposing use of an electronic format for submission of biographical sketches, including disclosure of all appointments. As currently envisioned, this will become effective in January 2020. We are also working to develop an electronic format for disclosure of current and pending support information.
- Foreign Government Talent Programs
- Finally, we are issuing a policy making it clear that NSF personnel and IPAs detailed to NSF cannot participate in foreign government talent recruitment programs. There is a risk that participation in foreign government talent recruitment programs by NSF personnel and IPAs will compromise the ethical principles that bind us. Moreover, such participation poses significant risks of inappropriate foreign influence on NSF policies, programs, and priorities, including the integrity of NSF's merit review process—risks we simply cannot accept.
Department of Energy [DOE]
DOE Directive O 486.1A, Foreign Government Sponsored or Affiliated Activities September 4, 2020
To ensure the continued flow of scientific and technical information consistent with the Department of Energy’s (DOE) broad scientific mission, while also ensuring protection of U.S. competitive and national security interests and DOE program objectives; preventing potential conflicts of interest, e.g., financial interests, conflicts of commitment, and outside employment, which may undermine the DOE research enterprise; and limiting unauthorized transfers of scientific and technical information. Cancels DOE O 486.1, dated 6-7-2019.
Science Article on DOE Policies February 8, 2019
DOE Directive regarding Foreign Government Talent Recruitment, June 7, 2019
DOE Order 486.1: "To ensure the continued flow of scientific and technical information consistent with the Department of Energy’s (DOE) broad scientific mission, while also ensuring protection of U.S. competitive and national security interests and DOE program objectives; and limiting unauthorized transfers of scientific and technical information."
Department of Defense [DOD]
"In his September 16, 2019. letter to the research community. Dr. Kelvin Droegemeier, Director of the White House Office of Science and Technology Policy (OSTP), described a new OSTP-led interagency Joint Committee on the Research Environment (JCORE).
DoD is an active participant in JCORE, and in its sub-committee on Research Security, which is initially focused on coordinating four lines of Federal effort:
- coordinating outreach and engagement
- disclosure requirements for participation in federally funded research
- best practices for academic research institutions
- methods for identification, assessment, and management of risk
This work will help agencies that fund Federal research to develop common standards for identifying and adjudicating conflicts of interest and conflicts of commitment from these disclosures. It will also help agencies that fund Federal research to clarify consequences for failing to make these disclosures."
Department of Defense Memo - Actions for the Protection of Intellectual Property, Controlled Information, Key Personnel and Critical March 20, 2019
“The National Defense Authorization Act (NOAA) for FY 2019, Section 1286, pages 443- 445, directs the Secretary of Defense to establish an initiative to work with academic institutions who perform defense research and engineering activities: 1. To support protection of intellectual property, controlled information, key personnel, and information about critical technologies relevant to national security; and 2. To limit undue influence, including through foreign talent programs, by countries to exploit United States technology within the Department of Defense research, science and technology, and innovation enterprise.”
Prohibition on Procurement of Foreign-Made Unmanned Aircraft Systems [DARS Tracking Number: 2020-O0015] [May 29, 2020]
Effective immediately, unless an exception applies or a waiver is granted, contracting officers shall not enter into or renew a contract for the procurement of—
- An unmanned aircraft system (UAS), or any related services or equipment, that—
- A system for the detection or identification of a UAS, or any related services or equipment, that is manufactured—
This prohibition does not apply to procurements for the purposes of: counter-UAS surrogate testing and training; or intelligence, electronic warfare, and information warfare operations, testing, analysis, and training.
This class deviation implements the procurement prohibition under section 848 of the National Defense Authorization Act for Fiscal Year 2020 (Pub. L. 116-92).
Department of State [DOS]
The State Department has issued a new determination under the Foreign Missions Act, requiring members of the Chinese diplomatic corps to notify the State Department when making visits to educational and research institutions, among other entities.
The Department’s determination requires U.S.-based personnel of the People’s Republic of China’s (PRC) foreign missions (including personnel at the embassy and consulates of the PRC and those on temporary assignments conducting official business for the PRC government) to notify the State Department’s Office of Foreign Missions in advance of:
- Official visits to educational institutions
- Official visits to research institutions, including National Labs
- Official meetings with state officials
- Official meetings with local and municipal officials
Chinese diplomats only need to inform the Department of these types of meetings. They do not need the State Department’s permission to visit educational institutions. The State Department indicated that the PRC government has chosen to prevent American diplomats from gaining access to Chinese campuses for educational and cultural programming, including programs that encourage students to study at American educational institutions. The new policy for PRC officials is a result of this situation.
NYT Article Under New Rule, Chinese Diplomats Must Notify State Dept. of Meetings in U.S.
National Aeronautics and Space Administration [NASA]
Gic 12-01 Class Deviation Implementing Nasa Restrictions On Funding Activities With The People's Republic Of China (Prc) Effective April 25, 2011
“NASA is restricted by specific applications of Section 1340(a) of The Department of Defense and Full-Year Appropriations Act, Public Law 112-10 (NASA's 2011 continuing resolution), and Section 539 of the Consolidated and Further Continuing Appropriation Act of 2012, Public Law 112-55 (NASA's FY 2012 appropriation) from using funding appropriated in the Acts to enter into or fund any grant or cooperative agreement of any kind to participate, collaborate, or coordinate bilaterally in any way with China or any Chinese-owned company, at the prime recipient level or at any subrecipient level, whether the bilateral involvement is funded or performed under a no-exchange of funds arrangement.”
Senate and NIH Correspondence
Sen. Chuck Grassley’s letter to NIH, Oct. 24, 2018: https://www.grassley.senate.gov/news/news-releases/chairman-grassley-seeks-transparency-nih-foreign-threats-research-grant-process
NIH response letter to Sen. Grassley, Dec. 21, 2018: https://www.grassley.senate.gov/sites/default/files/constituents/FR01%20WF%20376670%20Final%20Response.signed_0.pdf
Sen. Grassley’s response letter to NIH, Jan. 8, 2019: https://www.grassley.senate.gov/news/news-releases/grassley-receives-response-nih-foreign-threats-research-grant-process
Senate Committee on Finance
Senate Committee on Finance: "Grassley Probes Foreign Threats to Taxpayer-Funded Research at Defense Department," April 2, 2019: https://www.finance.senate.gov/chairmans-news/grassley-probes-foreign-th...
Senate Committee on Finance letter from Chuck Grassley to NSF Director France A. Córdova, April 15, 2019: https://www.finance.senate.gov/imo/media/doc/2019-04-15%20CEG%20to%20NSF%20(research%20threats)1.pdf
Congress
H.R.5515 - John S. McCain National Defense Authorization Act for Fiscal Year 2019115th Congress (2017-2018)
“SEC. 1286. INITIATIVE TO SUPPORT PROTECTION OF NATIONAL SECURITY ACADEMIC RESEARCHERS FROM UNDUE INFLUENCE AND OTHER SECURITY THREATS.
Department of Education
Higher Education Act Section 117 Foreign Gifts Reporting
The Higher Education Act of 1965, Section 117, is a federal law that requires most 2-year and 4-year postsecondary schools (whether or not they are eligible to participate in the Federal Student Aid programs) to report ownership or control by foreign sources and contracts with or gifts from the same foreign source that, alone or combined, have a value of $250,000 or more for a calendar year. Per the 60-day notice published on September 6, 2019, major changes are expected.
Department of Education announcement of New Reporting Portal June 22, 2020
Department of Education Foreign Gifts and Contracts Reporting Information Page
Federal Register Notice December 13, 2019
Federal Register Notice of Investigation and Record Requests, MIT, November 21, 2019
ECAS Compliance Alert October 8, 2019, Proposed Changes to Section 117
Federal Register Notice of Investigations into Section 117 Reporting Compliance
Federal Government Investigates Foreign Gifts to the University of Maryland
Department of Education July 3, 2019 Letter to ACE RE Section 117 Reporting
American Council on Education July 12, 2019 Letter to Department of Education
Department of Commerce
Huawei and 68 non-US Affiliates added to the Bureau of Industry and Security Entity List
“Entity List Additions of Huawei and 68 non-US Affiliates in Effect At 4:15 pm, May 16, 2019, a Federal Register Notice adding Huawei Technologies Co., Ltd. (Huawei) and 68 non-U.S. affiliates to the Entity List was published on the public inspection page of the Office of the Federal Register and went into effect. Unless authorized by the savings clause, all items subject to the Export Administration Regulations require a license to these parties, subject to a license review policy of presumption of denial.”
Huawei Entity List and Temporary General License Frequently Asked Questions
BIS added Huawei Technologies Co., Ltd. (Huawei) and its non-U.S. affiliates were added to the Entity List effective May 16, 2019, on the basis of information that provided a reasonable basis to conclude that Huawei is engaged in activities that are contrary to U.S. national security or foreign policy interests. This information included the activities alleged in the Department of Justice’s public Superseding Indictment of Huawei, including alleged violations of the International Emergency Economic Powers Act (IEEPA), conspiracy to violate IEEPA by providing prohibited financial services to Iran, and obstruction of justice in connection with the investigation of those alleged violations of U.S. sanctions. Effective August 19, 2019, BIS added another 46 non-U.S. affiliates of Huawei to the Entity List because they also pose a significant risk of involvement in activities contrary to the national security or foreign policy interests of the United States.
White House Executive Orders
Executive Order on Securing the Information and Communications Technology and Services Supply Chain May 15, 2019
“…further find that the unrestricted acquisition or use in the United States of information and communications technology or services designed, developed, manufactured, or supplied by persons owned by, controlled by, or subject to the jurisdiction or direction of foreign adversaries augments the ability of foreign adversaries to create and exploit vulnerabilities in information and communications technology or services, with potentially catastrophic effects, and thereby constitutes an unusual and extraordinary threat to the national security, foreign policy, and economy of the United States. This threat exists both in the case of individual acquisitions or uses of such technology or services, and when acquisitions or uses of such technologies are considered as a class.”
Executive Order prohibiting the entry of certain nationals of the PRC seeking to enter the United States pursuant to an F or J visa to study or conduct research in the United States determined to be detrimental to the interests of the United States.
Enforcement Activities
MIT Professor Arrested and Charged with Grant Fraud [January 14, 2021]
https://www.justice.gov/usao-ma/pr/mit-professor-arrested-and-charged-grant-fraud
A professor and researcher at Massachusetts Institute of Technology (MIT) was charged and arrested today in connection with failing to disclose contracts, appointments and awards from various entities in the People’s Republic of China (PRC) to the U.S. Department of Energy.
Harvard University Professor and Two Chinese Nationals Charged in Three Separate China Related Cases [January 28, 2020]
The Department of Justice announced today that the Chair of Harvard University’s Chemistry and Chemical Biology Department and two Chinese nationals have been charged in connection with aiding the People’s Republic of China. Dr. Charles Lieber, 60, Chair of the Department of Chemistry and Chemical Biology at Harvard University, was arrested this morning and charged by criminal complaint with one count of making a materially false, fictitious and fraudulent statement.
Visiting Stanford University Researcher Charged with VISA Fraud [June 21, 2020]
https://www.justice.gov/usao-ndca/pr/visiting-stanford-university-researcher-charged-visa-fraud
"The allegations describing the crime appear in an affidavit supporting the complaint filed on July 17, 2020. According to the affidavit, Song, 38, a Chinese national, entered the United States on December 23, 2018, using a J-1 non-immigrant visa. Song obtained the J-1 visa, a document “for individuals approved to participate in work-and study-based exchange visitor programs,” with an application she submitted in November 2018. In that application, Song stated that she had served in the Chinese military only from September 1, 2000, through June 30, 2011. She further stated that her employer was “Xi Diaoyutai Hospital” located at “No. 30 Fucheng Road, Beijing, 100142.” Song described herself in her visa application as a neurologist who was coming to the U.S. to conduct research at Stanford University related to brain disease."
Office of Science Technology Policy
NSPM-33: Presidential Memorandum on United States Government-Supported Research and Development National Security Policy [NEW January 19, 2021]
https://trumpwhitehouse.archives.gov/presidential-actions/presidential-memorandum-united-states-government-supported-research-development-national-security-policy/
JCORE: Recommended Practices for Strengthening the Security and Integrity of America’s Science and Technology Research Enterprise [NEW January 15, 2021]
Slides from the Federal Demonstration Partnership webinar featuring Dr. Kelvin Droegemeier from the White House Office of Science and Technology Policy. June 2020 https://www.whitehouse.gov/wp-content/uploads/2017/12/Enhancing-the-Security-and-Integrity-of-Americas-Research-Enterprise-June-2020.pdf.
NSTC Joint Committee on Research Environments July 2019 Update
OSTP Sends Open Letter to US Research Community on Foreign Influence Concerns
DHHS Office of Inspector General
Work Plan Update: Review of Institutions of Higher Education Grantees Receiving National Institutes of Health Awards May 2020
OIG has identified areas of potential risk at institutions of higher education receiving NIH awards such as inappropriate or unsupported charges to Federal awards, lack of financial conflict-of-interest polices, and deficiencies in internal control related to the financial management system. In addition, Congress, NIH, and Federal intelligence agencies have raised concerns about foreign threats to the integrity of U.S. medical research and intellectual property at institutions of higher education. Our objective will be to determine whether institutions of higher education (1) managed NIH awards to ensure allowability of costs in accordance with Federal and award requirements, and (2) met Federal conflict-of-interest requirements.
https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000467.asp
"The National Institutes of Health Has Limited Policies, Procedures, and Controls in Place for Helping To Ensure That Institutions Report All Sources of Research Support, Financial Interests, and Affiliations"
September 25, 2019 Audit (A-03-19-03003)
https://oig.hhs.gov/reports-and-publications/oas/nih.asp
STAT Article "NIH must better protect research from foreign influence, federal watchdog says"
Higher Education Organizations Science and Security Resources
Association of American Universities Science and Security Resource Document
Association of Public & Land-Grant Universities [APLU] and Association of American Universities [AAU] Actions Taken by Universities to Address Science and Security Concerns
Federal Law Enforcement Activity
Harvard Chemistry Chair Placed on Leave After Federal Gov. Charges He Hid Chinese Funding
Chemistry department chair Charles M. Lieber has been placed on an “indefinite” paid administrative leave after being charged in federal court with failing to disclose funding from the Chinese government, according to University spokesperson Jonathan L. Swain.
Havard Faculty Member Arrested, Affidavit link: https://assets.documentcloud.org/documents/6682460/Lieber-Affidavit-Harvard-Professor.pdf
MIT Professor Arrested for Grant Fraud: Defendant allegedly failed to disclose his work for the People’s Republic of China to U.S. Department of Energy
https://www.justice.gov/usao-ma/pr/mit-professor-arrested-and-charged-grant-fraud
Transparency in Disclosures of Foreign Relationships and Activities
For researchers, comprehensive disclosure ensures transparency and bolsters credibility, while on the other hand, failing to disclose can invite otherwise undue scrutiny, jeopardize funding or career opportunities, and could even result in legal prosecution. UC and its primary funders of sponsored research urge you, in the strongest terms possible, to disclose information about any and all other support, foreign components, or current and pending support, whether it’s provided through an organization or directly to you as an individual, as well as reporting all projects and activities that require a time commitment. Below we highlight a few of the notices from NIH and NSF on the importance of full disclosure.
NIH [PHS]
See NIH NOT-OD-21-073, NIH NOT-OD-19-114, NIH FAQs Other Support, Foreign Components, FCOI
Other Support includes all resources made available to a researcher in support of and/or related to all of their research endeavors, regardless of whether or not they have monetary value and regardless of whether they are based at the institution the researcher identifies for the current grant. This includes but is not limited to:
- Resources and/or financial support from all foreign and domestic entities, that are available to the researcher. This includes but is not limited to, financial support for laboratory personnel, and provision of high-value materials that are not freely available (e.g., biologics, chemical, model systems, technology, etc.). Institutional resources, such as core facilities or shared equipment that are made broadly available, should not be included in Other Support, but rather listed under Facilities and Other Resources.
- Consulting agreements, when the PD/PI or other senior/key personnel will be conducting research as part of the consulting activities. Non-research consulting activities are not Other Support.
- In-kind contributions, e.g. office/laboratory space, equipment, supplies, or employees or students supported by an outside source. If the time commitment or dollar value of the in-kind contribution is not readily.
Upcoming Changes to the Biographical Sketch and Other Support Format Page for Due Dates on or after May 25, 2021.
Effective May 25, 2021, NIH expects the following:- Supporting documentation, which includes copies of contracts, grants or any other agreement specific to senior/key personnel foreign appointments and/or employment with a foreign institution for all foreign activities and resources that are reported in Other Support. If the contracts, grants or other agreements are not in English, recipients must provide translated copies.
- Immediate notification of undisclosed Other Support. When a recipient organization discovers that a PI or other Senior/Key personnel on an active NIH grant failed to disclose Other Support information outside of Just-in-Time or the RPPR, as applicable, the recipient must submit updated Other Support to the Grants Management Specialist named in the Notice of Award as soon as it becomes known.
- Program Director/Principal Investigator or Other Senior/Key Personnel must sign the Other Support document to certify the accuracy of the information submitted. Each PD/PI or senior/key personnel must electronically sign their respective Other Support form as a PDF prior to submission.
What to disclose?
NIH Applicants must:
- List all positions and scientific appointments both domestic and foreign-held by senior/key personnel that are relevant to an application including affiliations with foreign entities or governments. This includes titled academic, professional, or institutional appointments whether or not remuneration is received, and whether full-time, part-time, or voluntary (including adjunct, visiting, or honorary).
- Report all resources and other support for all individuals designated in an application as senior/key personnel – including for the program director/principal investigator (PD/PI) and for other individuals who contribute to the scientific development or execution of a project in a substantive, measurable way, whether or not they request salaries or compensation. Information must be provided about all current support for ongoing projects, irrespective of whether such support is provided through the applicant organization, through another domestic or foreign organization, or is provided directly to an individual that supports the senior/key personnel’s research efforts.
- Report all current projects and activities that involve senior/key personnel, even if the support received is only in-kind (e.g. office/laboratory space, equipment, supplies, employees). All research resources including, but not limited to, foreign financial support, research or laboratory personnel, lab space, scientific materials, selection to a foreign “talents” or similar-type program, or other foreign or domestic support must be reported.
- Provide the total award amount for the entire award period covered (including facilities and administrative costs), as well as the number of person-months (or partial person-months) per year to be devoted to the project by the senior/key personnel involved.
When to disclose?
All pending support at the time of application submission and prior to award must be reported using “Just-in-Time Procedures” by providing all information indicated above. Applicants are responsible for promptly notifying NIH of any substantive changes to previously submitted Just-in-Time information up to the time of award, including “Other Support” changes that must be assessed for budgetary or scientific overlap. Further, if other support, as described as above, is obtained after the initial NIH award period, from any source either through the institution or directly to senior/key personnel, the details must be disclosed in the annual research performance progress report (RPPR). Post-award, recipients must address any substantive changes by submitting a prior approval request to NIH in accordance with the NIHGPS section on “Administrative Requirements—Changes in Project and Budget—NIH Standard Terms of Award.”
Foreign Components
What to disclose?
NIH requires recipients to determine whether activities it supports include a foreign component, defined as: The existence of any “significant scientific element or segment of a project” outside of the United States, in other words
- performance of work by a researcher or recipient in a foreign location, whether or not NIH grant funds are expended and/or
- performance of work by a researcher in a foreign location employed or paid for by a foreign organization, whether or not NIH grant funds are expended.
If a recipient determines that a portion of the project will be conducted outside of the U.S., the recipient then will need to determine if the activities are considered significant. If both criteria are met, then there is a foreign component. To aide with what may be considered significant, click on the FAQ link below.
When to disclose?
The addition of a foreign component to an ongoing NIH grant continues to require NIH prior approval, as outlined in the NIHGPS, Section 8.1.2 , Prior Approval Requirements.
Note: If an activity does not meet the definition of foreign component because all research is being conducted within the United States, but there is a non-U.S. resource that supports the research of an investigator and/or researcher, it must be reported as other support. For example, if a PD/PI of an NIH-funded grant has a collaborator outside of the U.S. who performs experiments in support of the PD/PI’s NIH-funded project, this would constitute a foreign component, regardless of whether the foreign collaborator receives funding from the PD/PI’s grant. Additional funding from a foreign source for the NIH-supported research of a PD/PI at a U.S. institution would not constitute a foreign component but would necessitate reporting as other support.
NSF
See Proposal and Award Policies and Procedures Guide: NSF Comments on Current and Pending Support Page II-23 June 2020
Current and Pending Support
Note: The requirement to use an NSF-approved format for preparation of current and pending support will go into effect for new proposals submitted or due on or after October 5, 2020. In the interim, proposers must continue to prepare this document in accordance with the guidance specified in the PAPPG (NSF 20-1). NSF, however, encourages the community to use the NSF-approved formats and provide valuable feedback as we enhance them for the October implementation.
Current and pending support information must be separately provided through use of an NSF-approved format, for each individual designated as senior personnel on the proposal. Current and pending support includes all resources made available to an individual in support of and/or related to all of his/her research efforts, regardless of whether or not they have monetary value. Current and pending support also includes in-kind contributions (such as office/laboratory space, equipment, supplies, employees, students. In-kind contributions not intended for use on the project/proposal being proposed also must be reported.
Current and pending support information must be provided for the proposed project, for ongoing projects, and for any proposals currently under consideration from whatever source, irrespective of whether such support is provided through the proposing organization or is provided directly to the individual.
The total award amount for the entire award period covered (including indirect costs) must be provided, as well as the number of person-months (or partial person-months) per year to be devoted to the project by the individual.
Concurrent submission of a proposal to other organizations will not prejudice its review by NSF, if disclosed. If the project (or any part of the project) now being submitted has been funded previously by a source other than NSF, information must be provided regarding the last period of funding.
Facilities, Equipment and Other Resources
The Facilities, Equipment and Other Resources document assesses the adequacy of the resources available to perform the effort proposed to satisfy both the Intellectual Merit and Broader Impacts review criteria. Proposers should describe only those resources that are directly applicable. Proposers should include an aggregated description of the internal and external resources (both physical and personnel) that the organization and its collaborators will provide to the project, should it be funded. Such information must be provided in this section, in lieu of other parts of the proposal (e.g., Budget Justification, Project Description). The description should be narrative in nature and must not include any quantifiable financial information. Reviewers will evaluate the information during the merit review process and the cognizant NSF Program Officer will review it for programmatic and technical sufficiency.
Although these resources are not considered voluntary committed cost sharing as defined in 2 CFR § 200.99, the Foundation does expect that the resources identified in the Facilities, Equipment and Other Resources section will be provided, or made available, should the proposal be funded. Chapter VII.B.1 specifies procedures for use by the grantee when there are postaward changes to objectives, scope or methods/procedures.
UC and Sponsor Policies
Conflict of Interest
Definitions:
A Financial Conflict of Interest exists when the Institution, through its designated official(s), reasonably determines that an Investigator’s Significant Financial Interest is related to a NIH-funded research project and could directly and significantly affect the design, conduct or reporting of the NIH-funded research.
"A conflict of interest in research exists when the individual has interests in the outcome of the research that may lead to a personal advantage and that might, therefore, in actuality or appearance compromise the integrity of the research." NAS, Integrity in Scientific Research
Relevant UC and Sponsor Policies
- UC NSF Policy
- UC NIH (PHS) Policy
- Institutional Conflicts of Interest RPAC Memo 11-05
- 45 CFR Part 50
- Financial Conflict of Interest NOT-OD-18-160 Investigator Disclosures of Foreign Interests
Conflict of Commitment
A conflict of commitment occurs when a faculty member’s outside activities interfere with the faculty member’s professional obligations to the University of California.
Relevant UC Policies
APM 025
“Conflict of Commitment and Outside Activities of Faculty Members” This policy defines which outside professional activities must be disclosed to the University, approved prior to engagement, and/or reported annually. This policy limits the amount of time a faculty member may devote to outside professional activities and describes the requirements when involving a student in outside professional activities. It defines activities as Category I, II, or III, and includes a Prior Approval form as well as an Annual reporting form. All faculty who are not members of a Health Sciences Compensation Plan (HSCP) are subject to this policy; however, faculty holding appointments of less than 50 percent time are not subject to the annual reporting and prior approval requirements.
APM 240
This policy is specific to academic deans, defined as a head of a division, college, school, or other similar academic unit, with administrative responsibility for that unit. APM 240-20c outlines additional restrictions on outside professional activities for Deans beyond the requirements of APM 025/671.
APM 246
“Faculty Administrators (100% Time)” Faculty Administrators who are appointed at 100% time are primarily responsible for administrative duties, but maintain their underlying faculty appointment. A Faculty Administrator holds a concurrent University faculty appointment. APM 246-20c outlines additional restrictions on outside professional activities for Faculty Administrators beyond the requirements of APM 025/671.
APM 671
“Conflict of Commitment and Outside Activities of Health Sciences Compensation Plan Participants” Faculty who are members of a Health Sciences Compensation Plan (HSCP) have additional requirements related to Outside Professional Activities, specific to income earned while engaged in outside professional activities. This policy also defines monitoring, compliance, and consequences for non-compliance. Faculty members appointed in Health Sciences schools that are not participants in the Health Sciences Compensation Plan are subject to APM-025.
UC Export Control Policy
The Export Control Policy sets expectations for roles and responsibilities, the designation of local Export Control Officer, and provides a framework for location export control programs. Federal export controls regulate items, information, and services, taken, sent or provided to other countries and technology shared with foreign nationals in the U.S. These controls are intended to protect U.S. economic interests and national security. The University maintains an open academic environment in support of its mission of teaching, research, and public service. At the same time, export control laws and regulations control the conditions under which certain information, technologies, and commodities can be shared.
Key elements of export control compliance
- Restricted party screening
- Considerations for hosting foreign visitors
- International shipping and hand-carry review
- Identify and safeguard controlled technology
The University is committed to complying with all U.S. export control laws. This Policy applies to all members of the University community, Faculty and Other Academic Appointees, Staff, Students and Non-employee participants in University programs and to all University activities.
Export control violations can result in civil and criminal penalties against the University, and/or assessment of fines or imprisonment of an individual.
For export control questions contact Marci Copeland, Systemwide Associate Director of Export Controls at (510) 987-9580 marci.copeland@ucop.edu or your location Export Control Officer.
FAQs
1. What is UC doing to address the issue?
ECAS has designed a series of compliance measures to address concerns related to foreign influence while ensuring our university’s commitment to fundamental research and maintaining our ability to apply academic freedom.
ECAS Strategy Foreign Influence Compliance Plan
This systemwide audit will identify and evaluate categories of federal grants for potential factors contributing to higher levels of risk related to foreign influence and review grants for compliance with requirements associated with foreign influence risk.
Each year, UC receives over $5 billion in research contracts and grants from a combination of federal, state, corporate, and non-profit sponsors. The majority of these awards $3.2 billion come from the federal government. Most of the University’s federal fudging is provided by the National Institutes of Health (NIH) and the National Science Foundation (NSF). With increasing frequency, federal agencies such as NSF are expressing concerns and inquiring about international engagements at research universities. As the largest recipient of funds from the NSF and NIH and in response to these and other developments, ECAS developed an Escalation Protocol to ensure that federal agency inquiries are appropriately and consistently tracked, escalated, reviewed and investigated across the UC system. This protocol provides a level of campus-wide and centralized visibility to connect separate but potentially related inquiries.
To address recent federal developments that increase the University’s risk associated with engagements with Huawei, the Office of Graduate Studies and ECAS worked closely with Vice Chancellors for Research to develop and outline restrictions on engagements with Huawei. The moratorium restricts any future engagements with Huawei, calls for the cessation of all pending projects, gifts, purchases or other engagements and requires locations to wind down existing agreements and consider risks associated with engagements with Huawei’s U.S. subsidiaries.
UC Memos
2. Who should I contact if I need clarification on how to comply with a policy, regulation, or generally need help understanding disclosure requirements?
Below you will find a list of links to campus resources such as Sponsored Projects Offices, Research Compliance Offices, Export Controls Offices. You may also contact our office, and we can either point you in the right direction for assistance at your campus or answer the question if it relates to ECAS work on foreign influence issues.
Office of Ethics, Compliance, and Audit Services Foreign Influence Contact:
Marci Copeland, Systemwide Associate Director of Export Controls - marci.copeland@ucop.edu
Links to UC Campus Resources, Websites, and External Tools
Links to UC Campus Research Compliance Offices
Links to UC Export Control Offices
Links to UC Sponsored Project Offices
UCSF Foreign Influence and Export Control
UCB Foreign Influences and Sponsored Projects April 16, 2019
UCI Guidance on Foreign Relationships/Affiliations
Federal Demonstration Partnership Meeting Materials on Foreign Influence
Foreign Influence Bench-marking Tracking Tool Developed by the University of Missouri
Links to other Institutions of Higher Education Foreign Influence websites/guidance
Harvard https://vpr.harvard.edu/files/ovpr-test/files/guidance_on_reporting_and_disclosure_requirements.pdf
PennState https://www.research.psu.edu/international_affiliations
